Table of Contents
1. Quantities, Units, Calcs.
1.1 Defining chemical engineering
1.2 Significant figures
1.3 Units and unit conversions
1.4 Properties
1.5 Temperature and temperature calculations
1.6 Pressure
1.7 Concentrations and fractions
1.8 Flow rates
1.9 Process unit: Pump
1.10 Quantities, units, and calculations problems
2. Material Balances
2.1 Process types and process units
2.2 Problem solving: 12 steps
2.3 Step 1: Process flow diagrams (PFDs)
2.4 Step 2: Defining a basis
2.5 Step 3: Systems and system boundaries
2.6 Steps 4-6: Material balance equations
2.7 Step 7: Writing extra equations
2.8 Step 8: Identifying unknowns
2.9 Steps 9-12: Solving equations and balances
2.10 Problem solving using all 12 steps for a multi-unit process
2.11 Recycle and bypass
2.12 Process unit: Distillation column
2.13 Process unit: Mixer
2.14 Process unit: Filter
2.15 Process unit: Evaporator
2.16 Process unit: Condenser
2.17 Material balances problems
3. Reacting Systems
3.1 Balancing a chemical reaction
3.2 Excess and limiting in chemical reactions
3.3 Fractional conversion
3.4 Extent of reaction
3.5 Yield and selectivity
3.6 Reaction equilibrium
3.7 Combustion reactions
3.8 Reaction with recycle
3.9 Process unit: Reactor
3.10 Process unit: Valve
3.11 Reacting system problems
4. Solids, Liquids, and Gases
4.1 Solid, liquid, and gas
4.2 Phase changes
4.3 Properties of steam
4.4 Ideal gases and ideal gas mixtures
4.5 Standard temperature and pressure
4.6 Vapor pressure and Antoine equation
4.7 Process unit: Compressor
4.8 Phase and property problems
5. Multiphase Systems
5.1 Raoult’s law
5.2 Bubble and dew point
5.3 Building two-component P-xy and T-xy diagrams
5.4 Flash
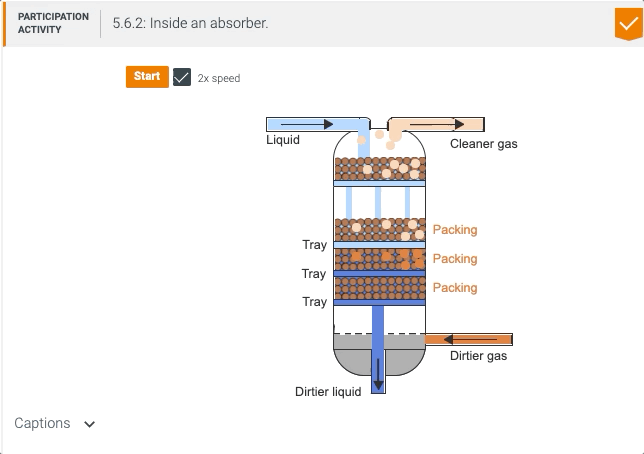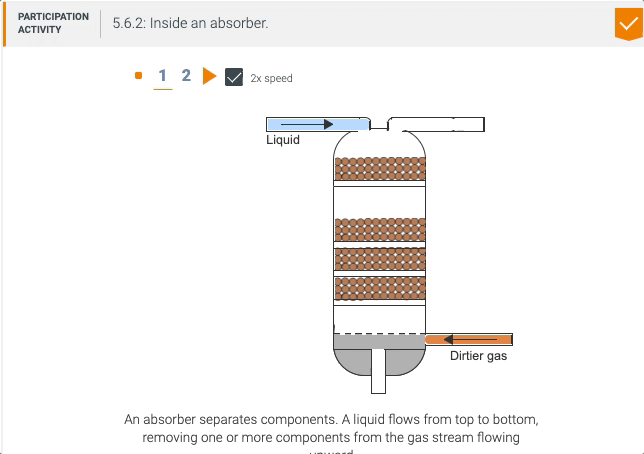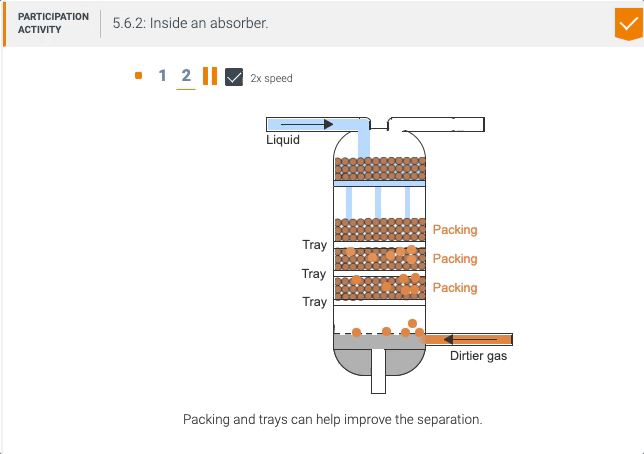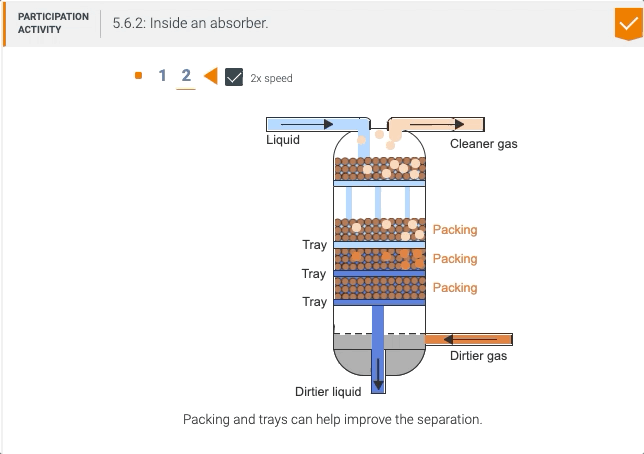
5.5 Process unit: Absorber
5.6 Process unit: Stripping column
5.7 Process unit: Flash tank
5.8 Multiphase problems
6. Energy Balances
6.1 Forms of energy
6.2 First law of thermodynamics and the energy balance
6.3 Enthalpy and tabulated enthalpies
6.4 Enthalpy and enthalpy paths
6.5 Heat capacity
6.6 Humidity
6.7 Process unit: Throttling valve
6.8 Process unit: Heat exchanger
6.9 Process unit: Turbine
6.10 Energy balance objectives and problems
7. Reaction + EB
7.1 Energy balances for reacting systems
7.2 Heat of reaction and Hess’s law
7.3 Heat of formation method
7.4 Combustion reactions and the energy balance
7.5 Simultaneous material and energy balances
7.6 Process unit: Fluidized bed reactor
7.7 Reaction + energy balances problems
8. Transient Systems
8.1 Transient material balances
8.2 Transient material balances with reaction
8.3 Transient energy balances
8.4 Process unit: Batch reactor
8.5 Transient problems
9. Spreadsheets
9.1 Spreadsheet basics
9.2 Spreadsheet formulas
9.3 Functions
9.4 Math functions
9.5 Logical and counting functions
9.6 Sorting and organizing data
9.7 Creating a chart
9.8 Trendlines
9.9 Solver and least squares fits
9.10 Error and statistics
9.11 Interpolation
9.12 Integration and numerical integration
9.13 Matrix functions
9.14 Systems of linear equations
9.15 Spreadsheet resources
10. Appendix
10.1 Unit Conversions
10.2 Nomenclature
10.3 Finding data and correlations
10.4 Periodic table of the elements
10.5 Physical properties
10.6 Properties of subcooled liquid water
10.7 Saturated water – temperature table
10.8 Saturated water – pressure table
10.9 Superheated vapor water/steam table
10.10 Vapor pressure
10.11 Heat capacity
10.12 Heat of solution for three common solutions
What You’ll Find In This zyBook:
More action with less text.
- Exceptionally-interactive introduction to Material and Energy Balances
- Over 140 animations and hundreds of interactive question sets
- Over 700 auto-graded, randomly generated challenge questions
- Adopters have access to a test bank with questions for every chapter
- An extensive appendix section, featuring data tables (available for free here)
The zyBooks Approach
Less text doesn’t mean less learning.
This zyBook provides a new, highly-interactive introduction to Material and Energy Balances, which is a first course in the exciting and growing field of Chemical Engineering. It is a complete replacement for existing textbooks on the topic and includes hundreds of interactive items proven to help students learn and stay engaged, and for which instructors often assign some homework points.
The basic concepts of mathematics, physics, and chemistry are applied to solving chemical engineering problems. Problem solving skills are developed for systems containing chemical reactions, multiphase and vapor-liquid equilibria, and recycle streams. Sketching process flow diagrams and calculating properties are also covered. Advanced topics include mass and energy balances for open, closed, transient, and reactive processes. Also, using a spreadsheet is detailed and offers basic skills used by many engineers.
Authors
Matthew Liberatore
Professor of Chemical Engineering, University of Toledo



